For Day 4 we performed the dirt analysis. It was fairly straight-forward and completed without much fanfare. The students once again enjoyed taking the information they collected and comparing it to the map that was provided and then matching up that information with what they found in the suspect bios.
Day 5 was DNA analysis day. We had a short conversation about what DNA is and I gave them a very simplified explanation of what a lab would do with a DNA sample that would lead to the results we were provided with in the kit. With all of that in mind, the actual analysis became more of a graph reading exercise than anything else. But, they did very well with it and enjoyed it quite a bit (I know one person mentioned it has being the favorite activity we did). Hopefully some small pieces of information will stick with them and will click into place when they're exposed to it again some day.
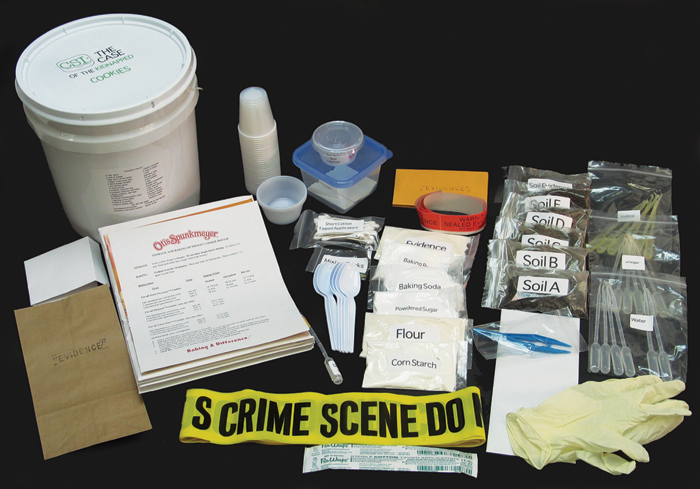
It was really great to have 5 weeks worth of library programming assembled into one place and ready to use. As I went through each of the activities, I thought about what/how I might do things if I were designing my own unit (and maybe I will sometime down the road.... add it to the bottom of the to-do list), and while there are changes I would like to make, it's hard to ignore the convenience of having everything ready-to-go. It would be a major investment of time to gather the supplies for a crime scene set-up and 4 days worth of lab activities, not to mention the time that would need to be spent planning a story line and creating all the other materials: suspect bios, maps, newspapers, ransom letters, etc. Many thanks to Educational Innovations for allowing us the opportunity to carry out such a comprehensive program while saving my sanity (my family is much appreciative)!
*****
The Case of the Kidnapped Cookies Kit was provided to me at no cost, for use with a group of students at my library. I was not compensated by Educational Innovations in any other way, and all opinions expressed are my own.